Chemistry of Mirroring

The chemistry of mirroring is simple but to get a reflective and tenacious mirror takes attention to detail.
Mirroring is not paint. It is a chemical reaction that occurs on the surface as the chemicals are poured or sprayed on.
Your first mirror will not be perfect. Mirroring is a skill. Skill is a combination of experience and knowledge.
You gain skill by practicing.


Cleaning
Cleaning is a chemical and mechanical process. The cleaning compound binds grease and dirt to the water molecules as you mix and push them around and then rinse them off the surface. Mirroring chemicals must be able to come into direct contact with the surface in order to form a coherent and adherent reflective surface. The surface must be clean for the mirroring to work.

Sensitizing
Sensitizers are the 'double-sided sticky tape' that binds the reflective metal layer to the surface. Much research has been done on how this works. It is thought that the sensitizer leaves a very thin layer of metallic tin on the surface, essentially tin-plating it. Thin in this case means about one atom thick. You can't see it but it is essential to the process. While the metal will deposit to some extent without sensitizing, it will be thin and patchy and will lift off easily. The amount of tin deposited is critical - too much will make the metal deposit too fast and black or gray spots will result. Too little and the metal layer will not form properly. Apply a generous layer of tin and then rinse it ALL off. What you need will stick to the surface so well you could not remove it with a fire hose. The tin that does not stick to the surface will contaminate the next layer.


Metallizing
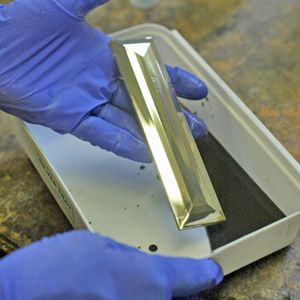
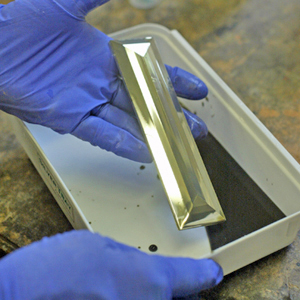
What we are trying to do here is to coat a surface with silver, copper, gold or galena. We do this by dissolving the metal in a strong acid. We add a complexer to keep it from depositing too fast and an activator to set the mixture to the right alkalinity. When you are ready to spray or pour it on the surface, you add a reducing agent which brings the metal out of solution as fine crystals which are attracted to the sensitized surface. If done at the right rate, a shiny metallic covering results. If done too fast, the particles clump together and form a black powder which wash off.
You can repeat the metallizing process to add a second layer on top of the first. There is a limit, however, and 2-3 passes is usually about it. Each layer is about 50 to 100 nm thick. That's nanometers - a millionth of a millimeter. By comparison, gold leaf is about one five-thousandth of a millimeter - about 200 times thicker. While this may not seem like much, the typical bathroom mirror is made industrially by this process and is usually double-coated.
Mixing Your Own Chemicals
Of course, you can buy your own chemicals and make your own mirroring solutions, but it might be cost effective. Some formulas need only a few grams of a chemical which can only be purchased in kilograms. Do you really want money tied up in supplies you will never use? We have spent 20 years (so far) testing various combinations of sensitizers, activators, reducers, and backing paints so they don't work against each other. We would be very interested in knowing if you have a better way of acheiving our results. Who knows, it could be the begining of a beautiful partnership.
History
The earliest mirrors were made of polished sheet metal which had to be re-polished frequently to remove oxidation and finger marks. A later development was called tin-amalgam which consists of rubbing liquid mercury onto a sheet of tin which is then pressed onto the glass with heavy weights. The tin-mercury process was so toxic that in 1835 the great German chemist Baron Justus von Liebig developed the simple, fast and non-toxic silver nitrate process we use today.